Case Study 1
Reengineering an Antiarrhythmic Drug for Improved Potency & Safety
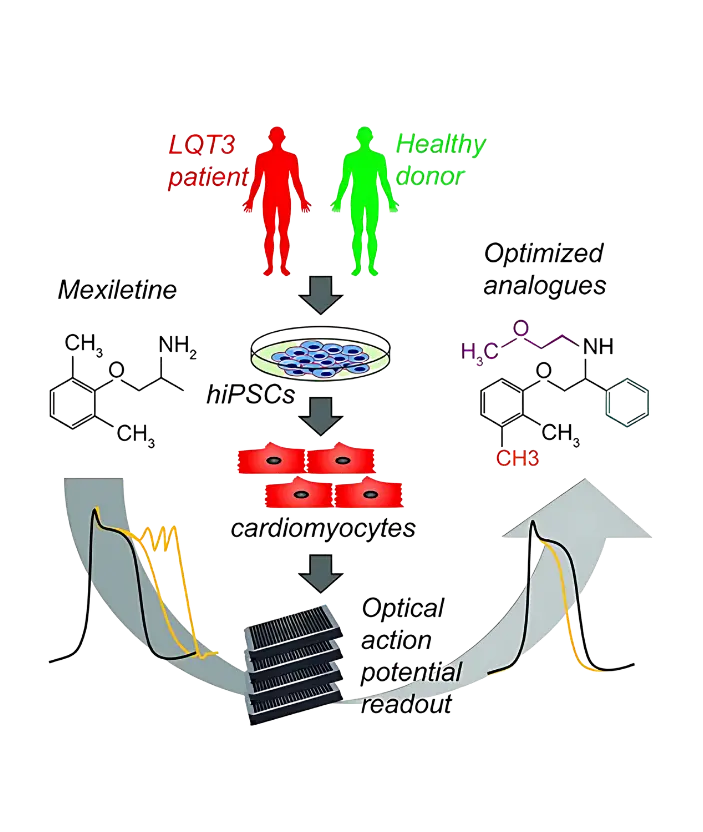
This case study explored reengineering of Mexiletine using the ValaDATE.AI™ platform to enhance its potency and safety. Mexiletine treats life-threatening ventricular arrhythmias and long QT syndrome, but it can cause side effects like irregular heartbeats and liver issues.
hiPSC Cell Generation
Human-induced pluripotent stem cell (hiPSC)-derived cardiomyocytes were generated from patients with long QT syndrome type 3 (LQT3) and healthy donors.
Screening Strategy
130 Mexiletine analogues were screened to identify compounds that shorten the action potential duration (APD) without causing proarrhythmic effects.
Functional Evaluation
The ValaDATE.AI™ platform was utilized to analyze APD shortening, potency, and efficacy, resulting in the identification of 17 promising analogues.
Lead Candidates
This study identified 4 lead candidates that showed improved therapeutic potential and reduced toxicity compared to Mexiletine.
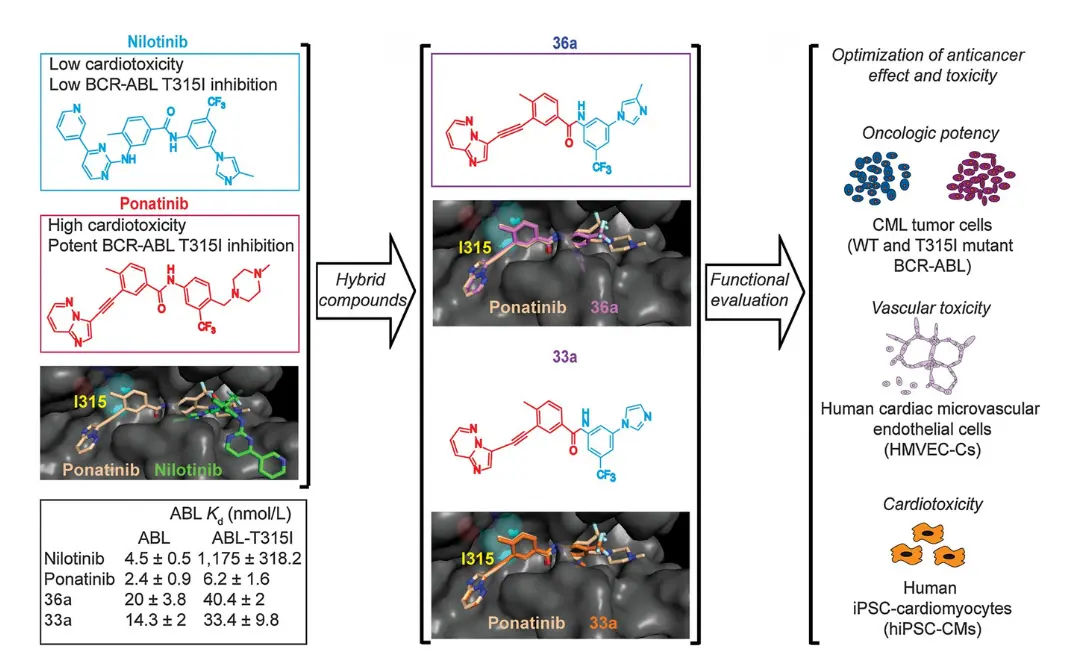
Case Study 2
Decrease the Cardiotoxicity Liability of an Oncology Drug
This case study utilized ValaDATE.AI™ to reduce the cardiotoxicity of Ponatinib, an oncology drug for Chronic Myelogenous Leukemia (CML) patients harboring the BCR-ABL T315I mutation. Although Ponatinib is effective, it can cause life-threatening cardiac side effects in some patients.
Chemical Diversification/Synthesis
Approximately 150 compounds were synthesized and evaluated to find a less cardiotoxic alternative to Ponatinib.
Functional Evaluation
The ValaDATE.AI™ platform was employed to identify compounds with high tumor inhibition and minimal cardiotoxicity.
Lead Candidates
Analogues 33a and 36a emerged as promising candidates, demonstrating tumor inhibition comparable to Ponatinib, while significantly reducing cardiotoxicity.
Anti-Cancer & Cardiotoxicity Assays
Compounds were tested for their ability to inhibit tumor growth and induce cardiotoxicity using a range of assays, including:
- Cell Proliferation: Tested on CML cells (both wild-type and T315I mutant BCR-ABL).
- Vasculogenesis: Assessed using human cardiac microvascular endothelial cells.
- Contractility: Evaluated using human induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs).